Site: New Orleans, Louisiana
Participating Hospital: Tulane University Hospital & Clinic
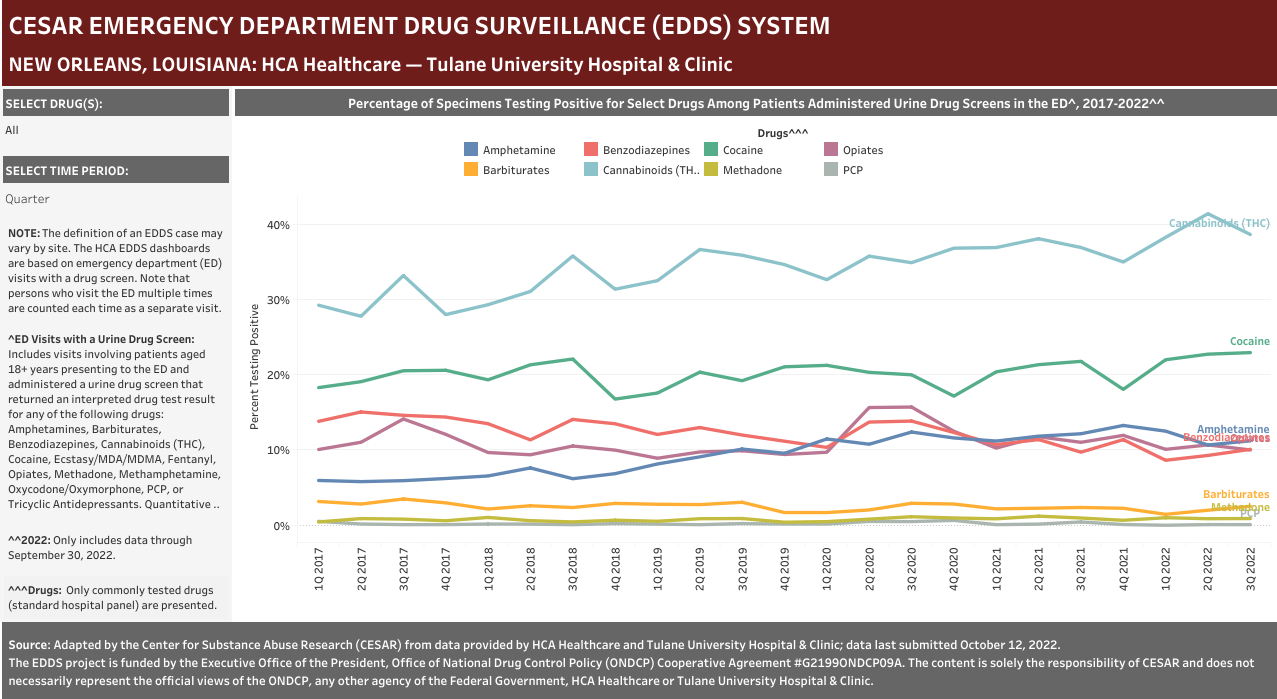
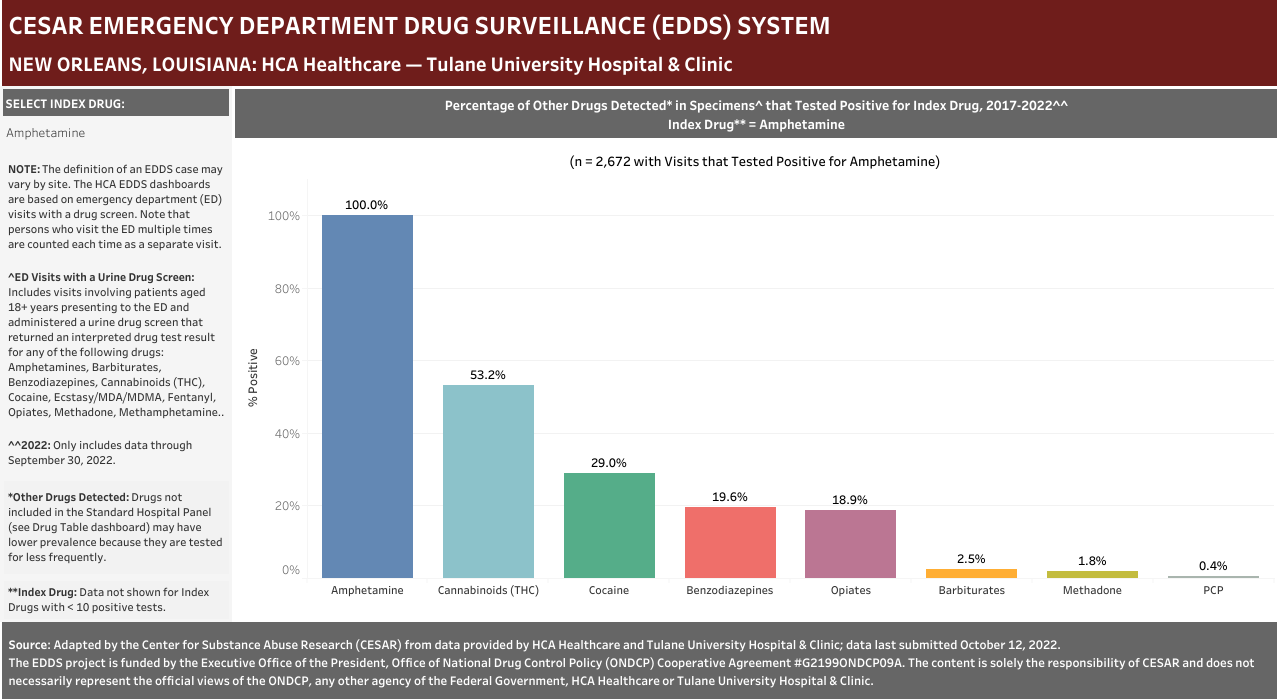
Case Definition: Hospital visits involving a patient aged 18 years or older presenting to the emergency department and administered a urine drug screen for any of the following drugs: Amphetamines, Barbiturates, Benzodiazepines, Cannabinoids (THC), Cocaine, Ecstasy/MDA/MDMA, Fentanyl, Opiates, Methadone, Methamphetamine, Oxycodone/Oxymorphone, PCP, or Tricyclic Antidepressants. Categories included in the analyses are positive, negative, and missing, but quantitative-only results are excluded. Note that each hospital did not necessarily screen for all of the drugs listed above—see Drug Table dashboard for hospital-specific drugs.
Notes about the Data:
- ED Visit: Persons who visit the ED multiple times are counted each time as a separate visit.
- Urine Drug Screen Administration in the ED: Toxicology screens are typically ordered when patients have symptoms that may be related to non-prescribed drug use. Most commonly, this includes altered mental status, suspected overdose and psychiatric evaluations.
- Urine Drug Screen Results: It is not possible for a urinalysis result to indicate whether the exposure to a drug was caused by personal use or if it was taken under a physician’s supervision.
- Opiate Screens: Opiate immunoassays are typically designed to detect morphine or codeine. However, other opiates such as hydrocodone, hydromorphone, oxycodone and oxymorphone may be detectable at higher concentrations. Other tests must be used to detect the presence of synthetic opioids such as fentanyl, methadone, and buprenorphine.
EHR Case Information Provided to EDDS: EDDS dashboards include hospital name, admission date (month/quarter/year), age, race, ethnicity, sex, and drug test results. Patient complaints, diagnoses and ICD-10-CM diagnostic codes, zip code, discharge disposition, COVID test results, and other administrative hospital variables to identify drug tests administered during ED encounter are also collected but not displayed in the EDDS dashboards.
Data Collection Beginning: January 2017
Data Collection Frequency: Twice a year.
The EDDS project is funded by the Executive Office of the President, Office of National Drug Control Policy (ONDCP) Cooperative Agreement #G2199ONDCP09A. The content is solely the responsibility of CESAR and does not necessarily represent the official views of the ONDCP, any other agency of the Federal Government, HCA Healthcare, or Tulane University Hospital & Clinic.
See also: