Site: Salt Lake City, Utah
Participating Hospital: University of Utah Hospital
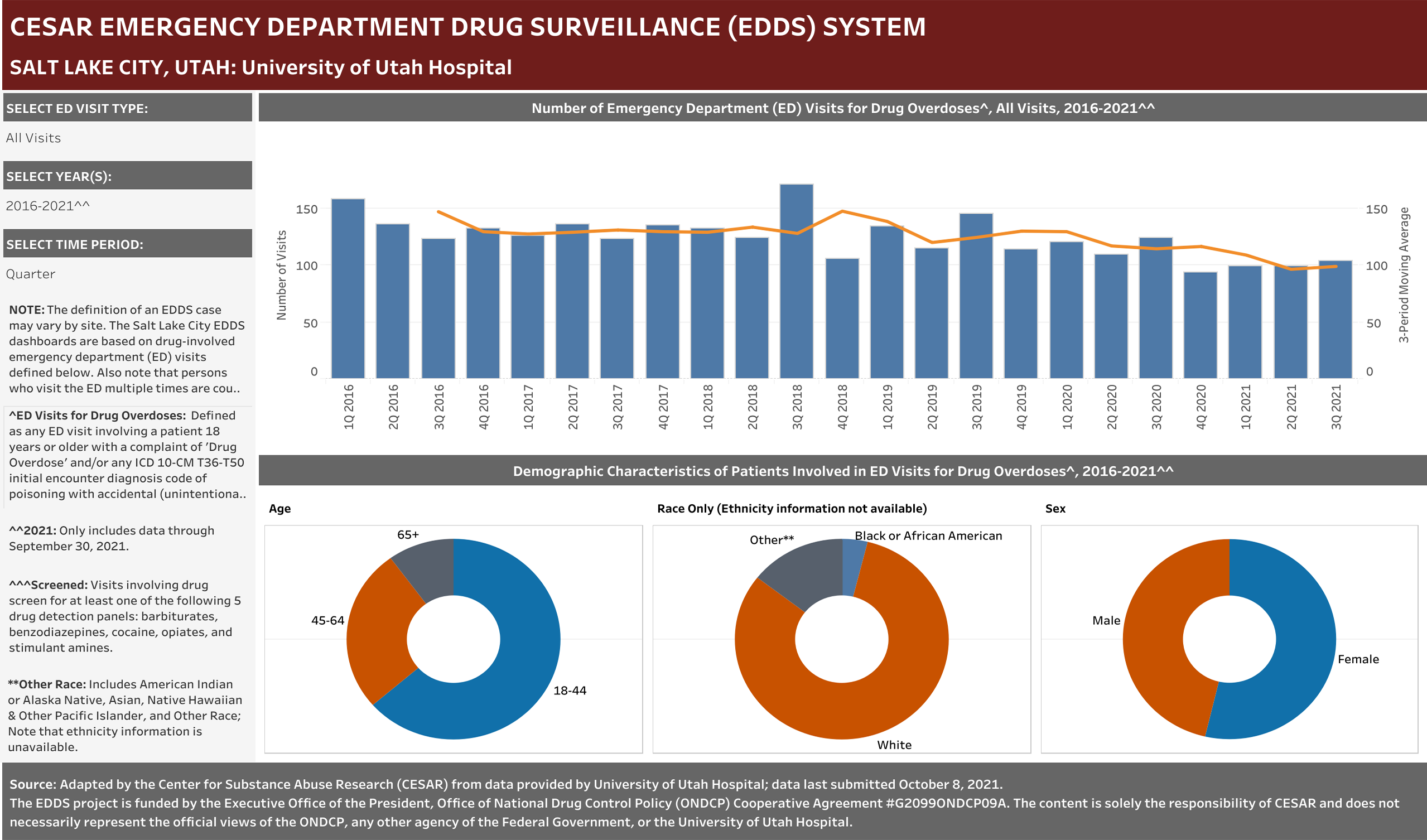
Case Definition: Hospital visits involving a patient aged 18 years or older presenting to the emergency department with a complaint of Drug Overdose and/or any ICD 10-CM T36-T50 initial encounter diagnosis code of poisoning with accidental (unintentional), intentional self-harm, or undetermined intent.” Only includes visits with initial encounter ICD-10-CM diagnosis codes of T36–T50 with a sixth character of 1, 2, or 4 (exceptions for T36.9, T37.9, T39.9, T41.4, T42.7, T43.9, T45.9, T47.9, and T49.9, which were included if the code had a fifth character of 1, 2, or 4) and a seventh character of “A” (initial encounter).
Any ED Visit with the term Drug Overdose term reported in any complaint field was included in the dataset. Any ED Visit with a Naloxone term reported in any complaint field would also be included in the dataset; however, no Naloxone terms were reported for this site.
Note that persons who visit the ED multiple times are counted each time as a separate visit.
EHR Case Information Provided to EDDS: Arrival date (month/year), departure date (month/year); ED disposition, patient complaint(s), diagnose(s) and ICD-10-CM diagnostic code(s), age, race, sex, zip code, and, when available, drug test results by panel ordered and COVID test results.
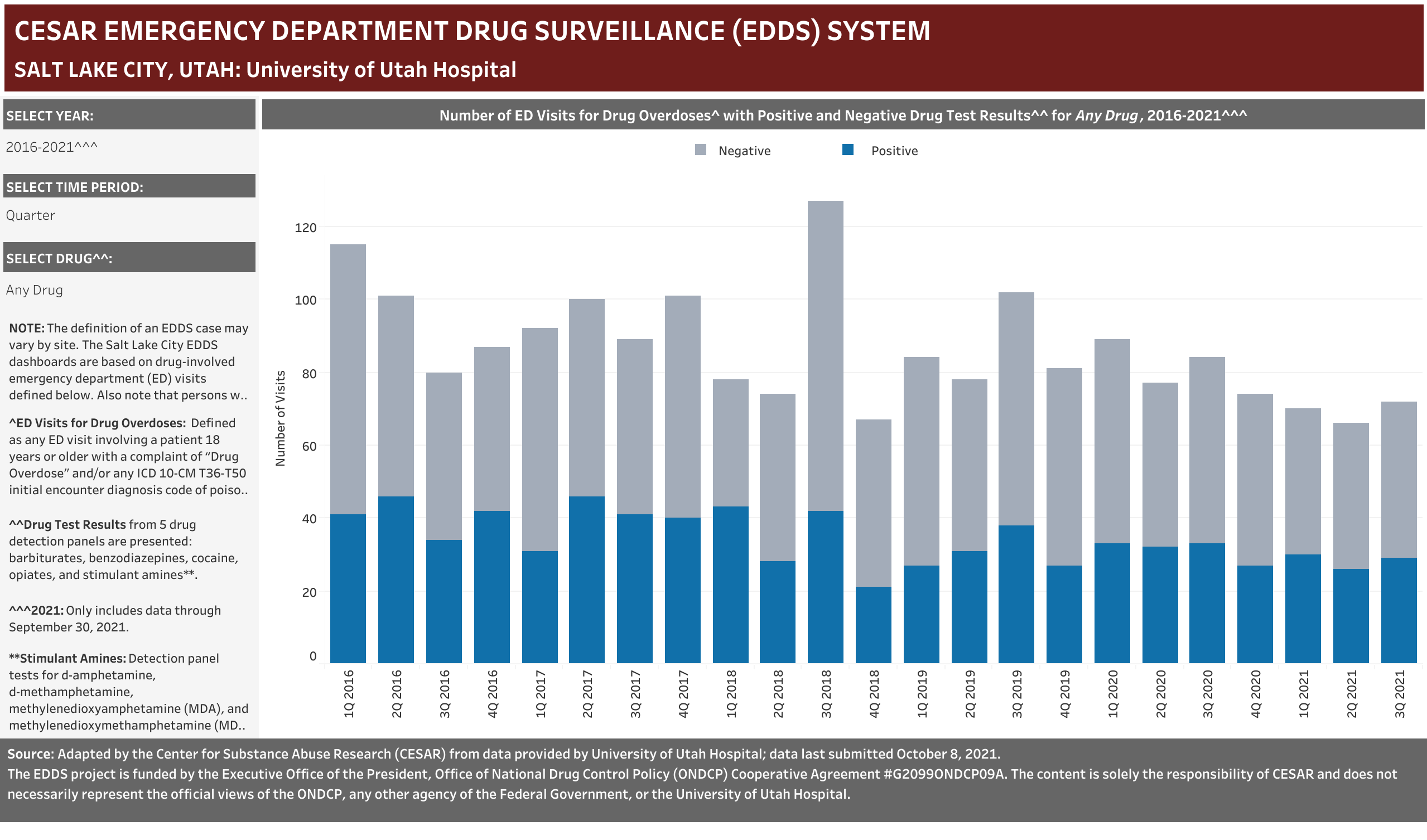
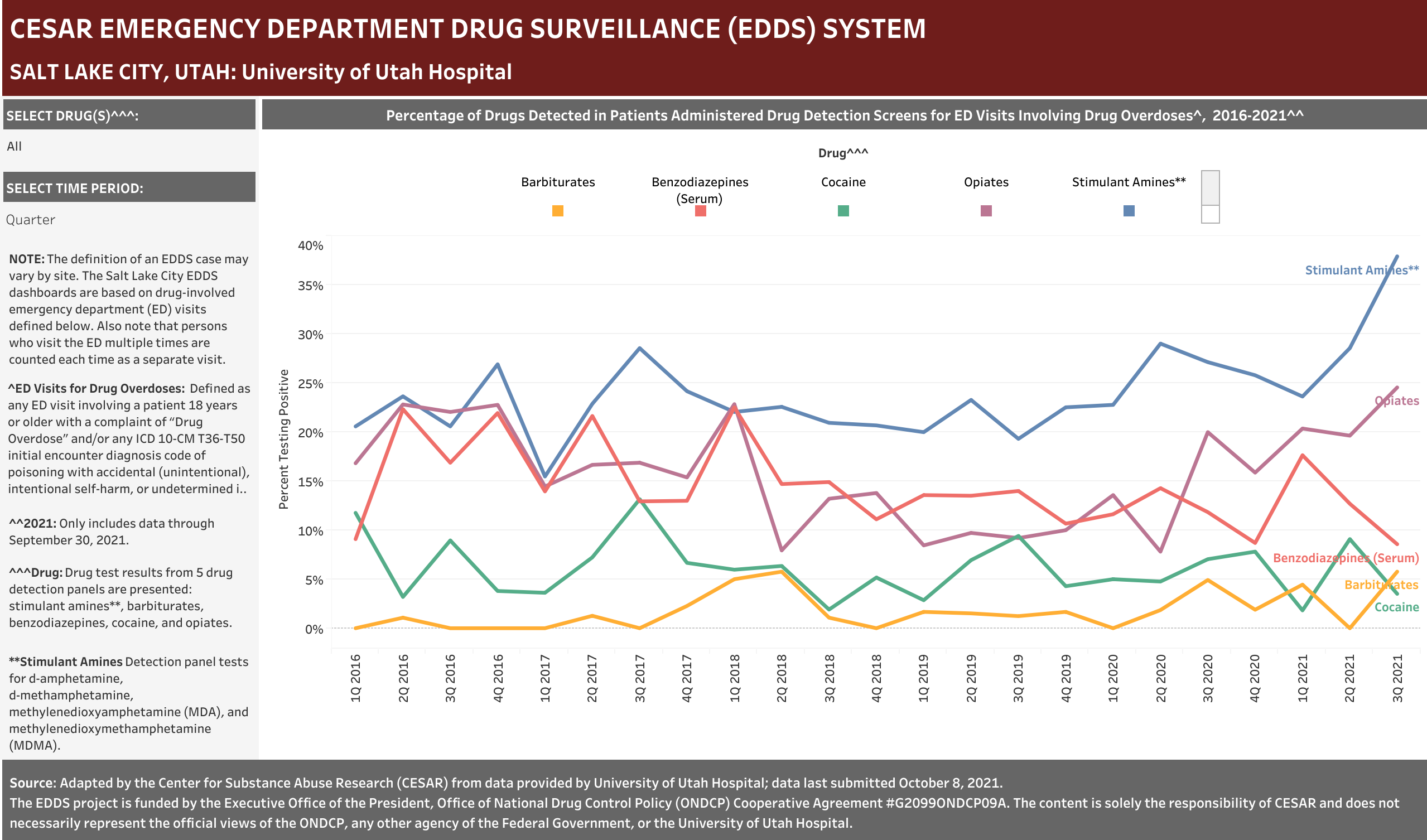
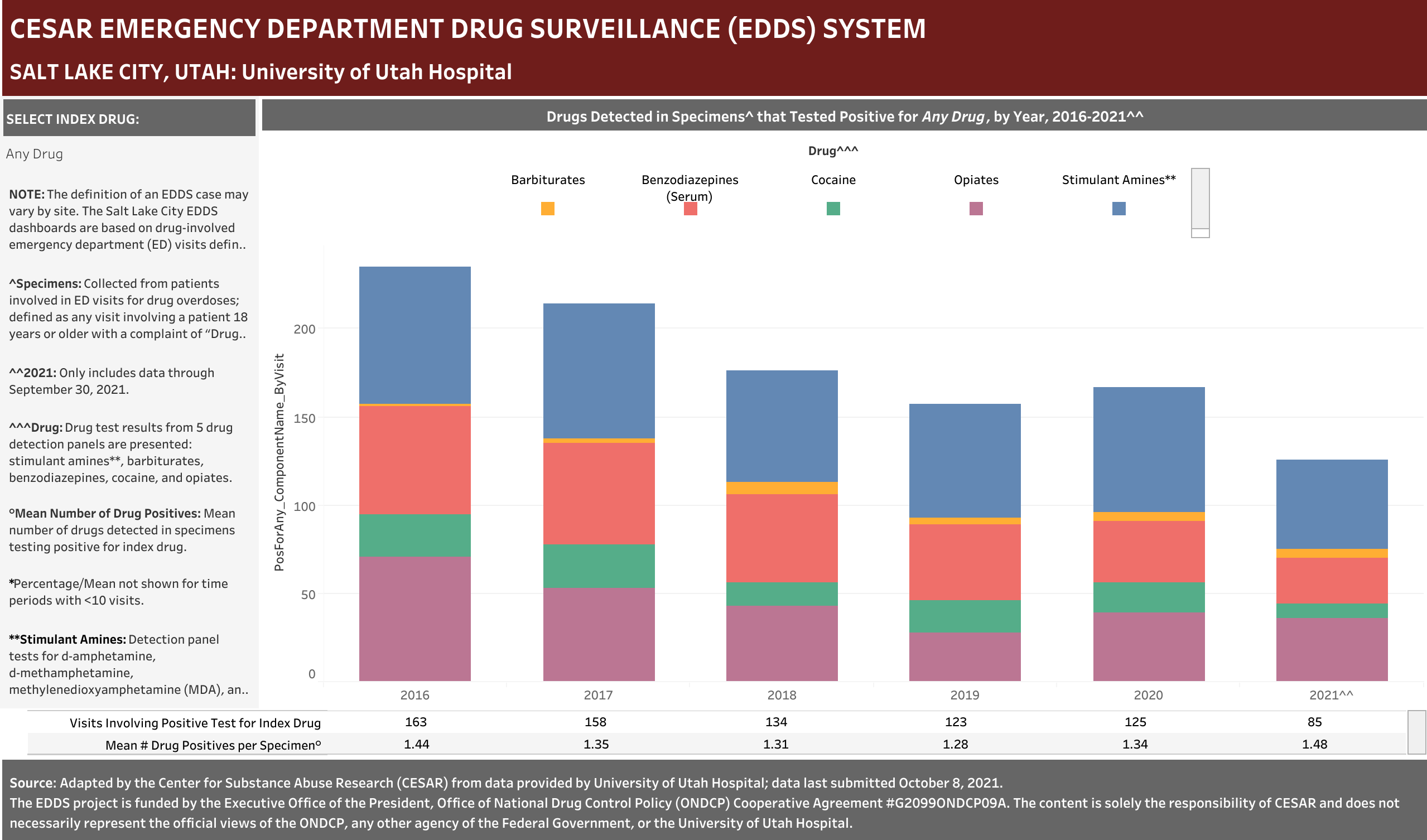
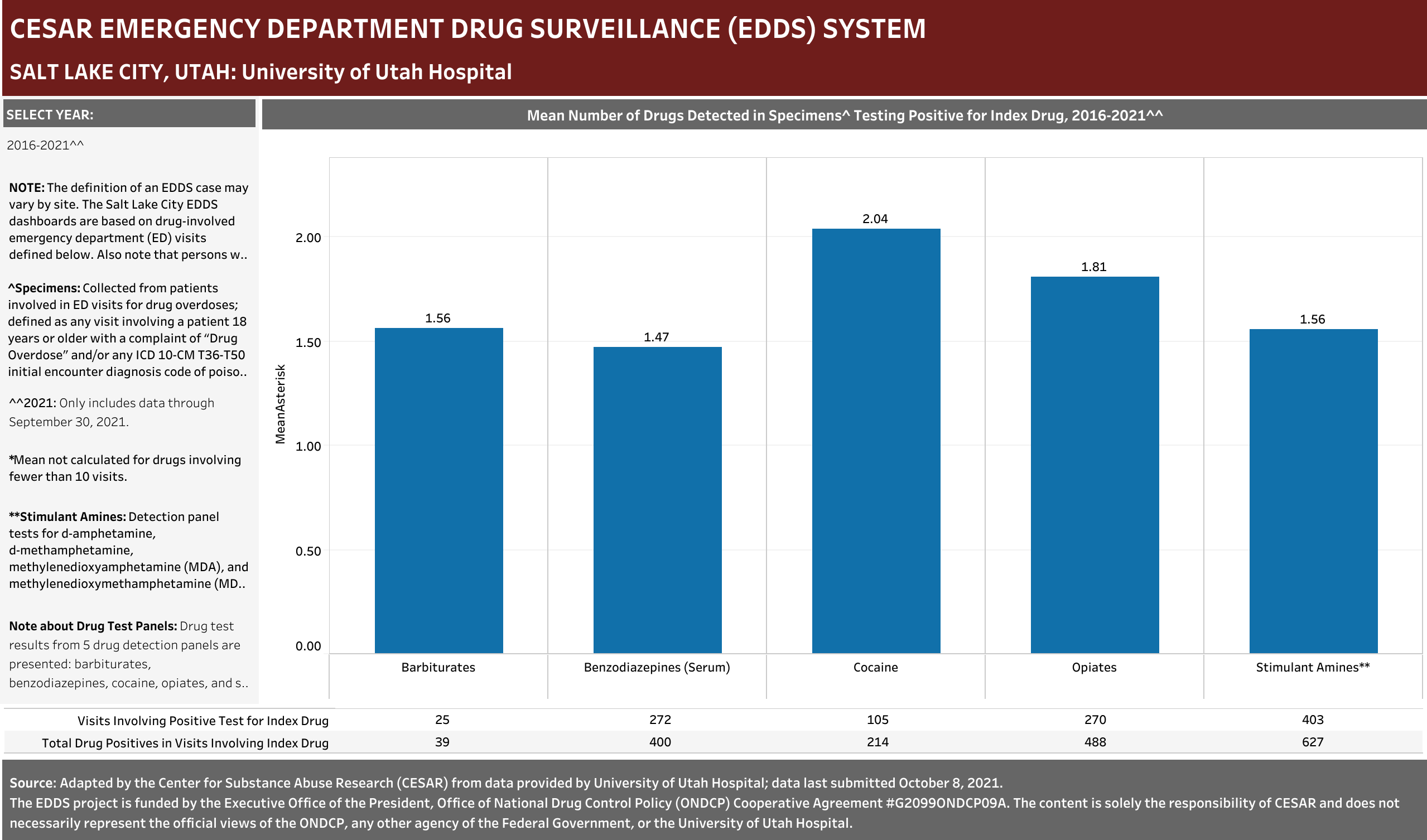
University of Utah Hospital typically screens specimens separately for five drugs: barbiturates, benzodiazepines, cocaine, opiates, and stimulant amines. The stimulant amines test detects amphetamine, methamphetamine, methylenedioxyamphetamine (MDA), and methylenedioxymethamphetamine (MDMA). The barbiturates, cocaine, opiates, and stimulant amines screens are conducted using urine and the benzodiazepines screen is conducted using blood (serum). Annually (2016-2021), at least one of these drug detection panels was administered in 98.6% of the ED drug overdose visits that were screened for a drug.
Data Collection Beginning: January 2016
Data Collection Frequency: Quarterly
The EDDS project is funded by the Executive Office of the President, Office of National Drug Control Policy (ONDCP) Cooperative Agreement #G2099ONDCP09A. The content is solely the responsibility of CESAR and does not necessarily represent the official views of the ONDCP, any other agency of the Federal Government, or the University of Utah Hospital.
See also: